Abstract

Background: Blockade of the cytokine IL-6 receptor by monoclonal antibodies, such as tocilizumab (TCZ), is a key strategy for the management of both CAR-T and T-cell dependent bispecific monoclonal antibody (TDB) induced cytokine release syndrome (CRS). Preclinical data indicate that inhibition of IL-6, IL-1, or TNF-α signaling prior to TDB administration can prevent development of CRS without any impact on anti-tumor activity (Li et al. Sci Transl Med 2019). To test this hypothesis in the clinic, a dedicated TCZ pretreatment arm was added to the ongoing GO39775 Phase I study (NCT03275103) of cevostamab, an FcRH5xCD3 TDB, to determine whether a single dose of TCZ administered prior to the first dose of cevostamab can reduce the incidence of CRS.
Methods: Patients (pts) who have RRMM for which no established therapy is available, appropriate or tolerable were enrolled in a dedicated TCZ pretreatment arm. Cevostamab was administered intravenously (IV) in a 21-day Cycle (C). A single 3.6mg priming dose was given on Day (D) 1 of C1 followed by a target dose of 90mg on C1D8 and then C2D1 and beyond. A single IV 8mg/kg dose of TCZ was administered as a pretreatment on C1D1 two hours prior to the administration of cevostamab. Patient data from the previously enrolled non-TCZ 3.6/90mg dosing arm served as a retrospective comparator. Pts in both arms received corticosteroid, antihistamine and acetaminophen premedication prior to cevostamab. CRS is reported using ASTCT criteria (Lee et al. Biol Blood Marrow Transplant 2019).
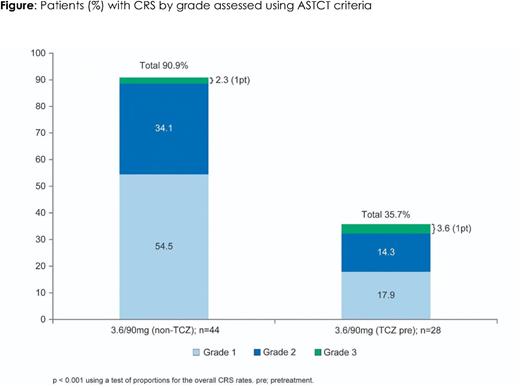
Results: At data cut-off (March 8, 2022), 28 pts had enrolled in the TCZ pretreatment arm (median age: 64.5 years) and 44 pts in the non-TCZ comparator (median age: 63.0 years). Median prior lines of therapy were 4 (2.0-7.0) and 6 (2.0-11.0) in the TCZ pretreatment and non-TCZ arms, respectively. Twenty-two pts (78.6%) were triple-class refractory and 12 pts (42.9%) were penta-refractory in the TCZ pretreatment arm, vs. 38 (86.4%) triple-class refractory and 32 (72.7%) penta-refractory pts in the non-TCZ arm. Pts in the TCZ arm had a median time on study of 4.7 months (range: 0.3-11.3) vs. 13.1 months (range: 0.2-30.8) in the non-TCZ arm. All patients in the TCZ arm experienced at least one adverse event (AE). Overall, 35.7% of pts in the TCZ arm experienced CRS as compared to 90.9% in the non-TCZ 3.6/90mg group (see figure). One patient in each group experienced G3 CRS and no G4/G5 events were observed. Fewer pts required TCZ to treat CRS (5/28) in the TCZ arm than the non-TCZ arm (16/44). Only two pts on the TCZ arm had more than one CRS event (vs. 12/44 non-TCZ) and no CRS events were observed at C2 and beyond (vs. 5/44 non-TCZ). Rates of non-CRS AEs (notably thrombocytopenia, infections, and liver enzyme elevations) in the TCZ arm were similar to the non-TCZ arm with the exception of neutropenia (Trudel et al. ASH 2021). In the TCZ arm 64.3% of pts experienced G3/G4 neutropenia as compared to 38.6% in the comparator arm. In both arms, neutropenia events were reversible, manageable with growth factor as indicated, and none led to the discontinuation of cevostamab.
Consistent with preclinical data, no apparent negative impact on anti-tumor activity was observed. The overall response rate in the TCZ arm with 90mg target dose was 50% (13/26) [95% CI: 28.9-71.2] compared to 37.2% (16/43) [95% CI: 21.6-52.8] in pts receiving 90mg without TCZ pretreatment. Very good partial response or better (VGPR+) rates were 26.9% vs. 25.6%, respectively. Available pharmacodynamic data did not demonstrate any apparent change in measures of T-cell activation and proliferation in pts receiving TCZ pretreatment. Consistent with the known mechanism of action of TCZ, levels of C-reactive protein, a downstream product of hepatic IL-6 activation, were heavily suppressed in the vast majority of pts in TCZ arm suggestive of near-complete inhibition of the IL-6 signaling pathway.
Conclusions: Clinical data from the GO39775 study shows for the first time that TCZ pretreatment can significantly reduce the risk of developing TDB-induced CRS without an apparent impact on anti-myeloma activity. The data support additional investigation of the use of anti-cytokine pretreatment with the goal of substantially reducing the frequency and potentially the severity of CRS.
Disclosures
Trudel:Genentech: Research Funding; Sanofi: Honoraria; Forus: Consultancy; Janssen: Honoraria, Research Funding; AstraZeneca: Honoraria; Karyopharm: Honoraria; Takeda: Honoraria; Pfizer: Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; GlaxoSmithKline: Consultancy, Honoraria, Research Funding. Bahlis:AbbVie, Amgen, Bristol Myers Squibb, Celgene, Forus, Janssen, Genentech, GSK, Karyopharm, Novartis, Pfizer, Takeda, Sanofi: Consultancy; Pfizer: Research Funding. Spencer:Amgen: Consultancy, Honoraria; Haemalogix: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria. Kaedbey:Sanofi: Other: Advisory boards; FORUS Therapeutics: Other: Advisory boards; Pfizer: Other: Advisory boards; Beigene: Other: Advisory boards; BMS: Honoraria, Other: Advisory boards; Janssen: Honoraria, Other: Advisory boards; Jewish General Hospital, Montreal, QC, Canada: Current Employment; BMS. Janssen: Honoraria; Janssen, BMS, Sanofi, FORUS, Beigene, Pfizer: Membership on an entity's Board of Directors or advisory committees; BMS. Janssen: Honoraria; Janssen, BMS, Sanofi, FORUS, Beigene, Pfizer: Membership on an entity's Board of Directors or advisory committees. Rodriguez Otero:BMS: Consultancy; Sanofi: Consultancy, Speakers Bureau; Pfizer: Consultancy; GSK: Consultancy, Speakers Bureau; Amgen: Speakers Bureau; BMS-Celgene: Speakers Bureau; Regeneron Pharmaceuticals, Inc.: Speakers Bureau; Amgen, Sanofi, GSK, Janssen, BMS-Celgene, Regeneron: Speakers Bureau; Janssen, BMS, Sanofi, Pfizer, GSK.: Consultancy; Janssen: Consultancy, Speakers Bureau; Hematology Clínica Universidad de Navarra: Current Employment. Harrison:Haemalogix: Membership on an entity's Board of Directors or advisory committees; Abbvie, Amgen, Celgene/BMS, GSK, Janssen Cilag, Novartis, Roche Genentech, Eusa: Speakers Bureau; Abbvie, Amgen, Celgene/BMS, GSK, Janssen Cilag, Novartis, Roche Genentech, Haemalogix, Eusa, Terumo BCT: Honoraria; Celgene/BMS, GSK, Janssen Cilag, Haemalogix: Research Funding; Abbvie, Amgen, Celgene/BMS, GSK, Janssen Cilag, Novartis, Roche Genentech, Haemalogix, Eusa, Terumo BCT: Consultancy. Wong:Genentech/Roche: Current Employment; CTMX and BMRN: Current equity holder in publicly-traded company; Patents but no royalties: https://patents.justia.com/inventor/chihunt-wong: Patents & Royalties: https://patents.justia.com/inventor/chihunt-wong; "For Henry": https://www.forhenryahc.org/: Membership on an entity's Board of Directors or advisory committees. Goodman:Genentech, Inc.: Current Employment; Roche shares and stock-settled appreciation rights: Current equity holder in publicly-traded company. Nakamura:F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company; Genentech, Inc.: Current Employment. Choeurng:Genentech: Current Employment, Current equity holder in publicly-traded company. Cooper:Genentech: Current Employment. Mateos:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal